FDA Signs Off on Bivalent COVID Shot as Fourth Dose for Kids Under 5 With Literally No Data
The FDA showed once again that it doesn't care about your kids after it signed off on infants getting fourth doses of the clot shots. Babies could have eight COVID vaccine exposures by age 10 months.
The U.S. Food and Drug Administration (FDA) hit a new low on Tuesday, authorizing a fourth COVID vaccine dose for the nation’s smallest children.
The agency authorized a booster dose of Pfizer’s experimental bivalent COVID-19 vaccine for kids under five years old who previously received the three-dose primary series. Theoretically, an infant could have four COVID vaccine doses by the time it is 10 months old and eight total exposures if you count vaccination during pregnancy and breastfeeding—as we know, COVID vaccine mRNA passes through the placenta and breastmilk to an infant.
Now, one might think this decision was based on loads of data showing a fourth dose is safe and effective, right? But, no, this is the FDA we’re talking about. This is the agency that signed off on bivalent boosters in the first place with no human clinical trials, efficacy data using an entirely different vaccine, and safety data of eight mice.
The FDA continuously uses bad or no data to make sweeping recommendations for America’s children that could result in horrible adverse events you and I have to pay for — and Pfizer is off the hook for.
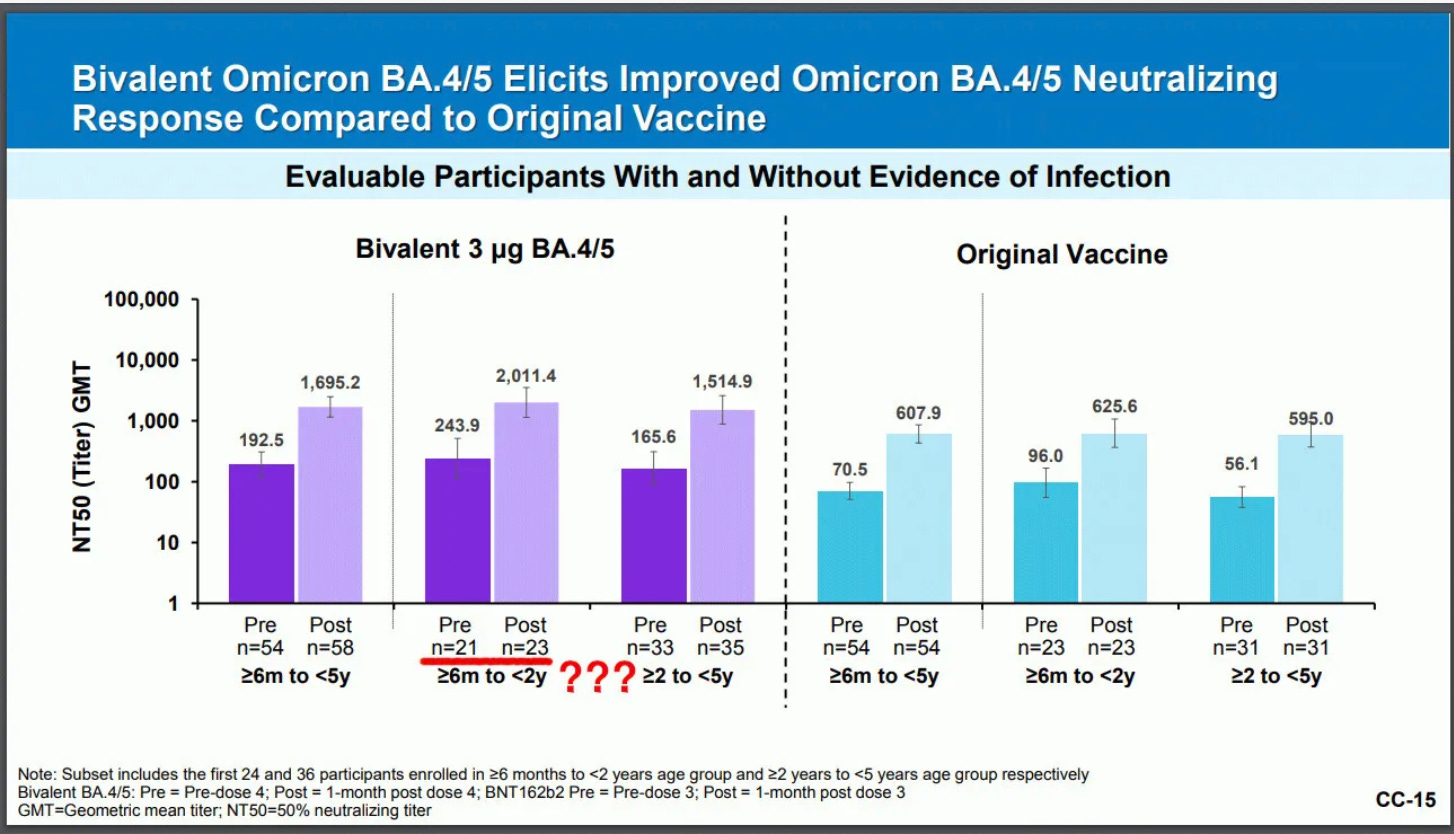
The FDA said in a press release it utilized previous vaccine efficacy data—which we all know is laughable at best, and a tiny “clinical trial” (if we can even call it that) of 60 children.
You cannot make this up. Pfizer had roughly 24 children in the 6-month to 23-month age group in its clinical trial and 36 participants 2 years through age 4.
“Among 24 participants 6 months through 23 months, the most common side effects included irritability, drowsiness, injection site redness, pain and swelling, decreased appetite, fatigue, and fever. Among 36 participants 2 years through 4 years of age, the most common side effects included fatigue, injection site pain, redness and swelling, diarrhea, vomiting, headache, joint pain, and chills.”
We don’t know the other side effects, nor are these samples large enough to detect what adverse events we may see in the real world. In fact, we don’t know the actual results of this “clinical trial” because the results haven’t been published.
We only know what the FDA said in its press release and what Pfizer presented during a Jan. 26 meeting of the agency’s vaccine advisors. During the meeting, Pfizer wasn’t even consistent with the number of children who participated in its trial.
As writer Igor Chudov recently pointed out, there were a different number of participants listed in the pre and post-vaccine groups for the bivalent shot. Of course, none of the geniuses advising the FDA caught this or pointed it out.
On Jan 11, 2023, U.S. News reported that two studies published in the New England Journal of Medicine found updated COVID-19 bivalent boosters targeting Omicron subvariants didn’t appear to provide any better protection than the original shots containing the non-existent Wuhan strain.
Together, the two studies “suggest that with this rapidly evolving virus, vaccines developed for different strains are not going to add a huge difference in terms of protection,” said Dr. Greg Poland, director of the Mayo Clinic’s Vaccine Research Group.
“It appears that human immune systems imprint after exposure to the first mRNA COVID vaccines” and are “primed to respond to aspects of the original COVID-19 strain that are shared by all the variants, rather than the novel mutations sported by newer variants.”
“It may be that people’s immune systems are so primed to respond to the ancestral strain spike protein that a reformulated booster is unable to fully stimulate the immune system because it has been ‘imprinted’ by the original version of the virus,” said Dr. Amesh Adalja, senior scholar at Johns Hopkins Center for Health Security.
Numerous studies have been published since showing drinking Kool-Aid to prevent COVID is more effective than getting a bivalent booster. Alas,’ here we are.
We all know that the original vaccines do not work. They do not prevent infection or transmission, nor have legitimate peer-reviewed or real-world data shown they reduce the severity of COVID. The only thing COVID vaccines do is temporarily boost antibody levels, which the FDA has already said is not indicative of immunity.
We also know that the vast majority of children have had COVID-19 and have developed natural immunity to the virus — and that for those who haven’t, their risk of severe COVID is exceedingly low.
Yet, the FDA continues to perpetuate its COVID vaccine quackery to manipulate parents into signing their children up for endless doses of mRNA and spike protein—the full effects of which we may not see for decades.



We know from this that something else is going on.
Money laundering Politics has let our once free country be taken over by nonelected alphabet soup organizations.
The FDA may have had good intentions once upon a time but now it is right there with the CIA(They even destroy one another), FBI (Russiagate), CDC, WHO(planning world take over), USDA (farmers can't sell products), BLM (Bureau that confiscates land and sells Uranium to Russia), IRS(which was used by Obama to target political opponents) FEMA(which sent 2 personal friends to prison over a lie) and the list goes on and on.
Absolutely no HEALTH reason to Jab anyone (except maybe as evidence shows it is to destroy health)!
If about half the jabs are a placebo and as Cancer Doctors claim there is a 20 fold increase in CANCER from the TOTAL jabbed then the 20 fold increase is now only among the real victims it means a far greater group will get boosters of the real thing!
Have no fear cause NOW they have a Cancer thing out to earn more bucks for the famous Fauci jab maker friend!
I went online and counted the obituaries for one funeral home and there IS a big increase. 2020=110, 2021=147, 2022=137, and there aren't usually obituaries for babies and stillborns.